Table of Contents
- Key Points
- Alpha, Beta, and Gamma Radiation
- Natural Occurrence and Detection
- Marie Curie’s Contributions
- Survey Note: Detailed Analysis of Radioactivity, Radioactive Materials, and Marie Curie’s Contributions
- Introduction to Radioactivity and Radioactive Materials
- Uranium-238 Decay Chain: Correction and Analysis
- Properties of Radioactive Elements
- Types of Radiation: Alpha, Beta, and Gamma
- Natural Occurrence and Detection
- Marie Curie’s Biographical Details and Contributions
- Comparative Analysis and Unexpected Details
- Tabular Summary of Key Works and Concepts
- Conclusion
- Key Citations
Key Points
- Research suggests radioactivity involves spontaneous emission from unstable atomic nuclei, with uranium-238 decaying through 14 steps to lead-206, not 18 as stated.
- It seems likely that radioactive materials are natural, with most exposure from cosmic rays and some from rocks, detected by Geiger counters.
- The evidence leans toward Marie Curie discovering radioactivity and elements like polonium and radium, winning two Nobel Prizes.
Alpha, Beta, and Gamma Radiation
Radioactivity emits three main types of radiation: alpha, beta, and gamma. Alpha particles are large and positively charged, easily blocked by paper or clothing. Beta particles, negatively charged, can penetrate deeper, stopped by materials like aluminum. Gamma rays, high-energy electromagnetic waves, pass through most materials but are blocked by dense substances like lead, often used in protective vests in hospitals.
Natural Occurrence and Detection
Most radioactive materials occur naturally, with significant exposure from cosmic rays (sun and stars) and some from rocks and minerals like uranium in brick or concrete houses, increasing radiation compared to wooden houses. The Geiger counter, a well-known device, detects ionizing radiation like alpha, beta, and gamma rays using ionization effects.
Marie Curie’s Contributions
Marie Curie, born in 1867, discovered radioactivity and, with her husband Pierre, identified polonium and radium from pitchblende. She was the first woman to win a Nobel Prize (Physics, 1903) and the first person to win two (Chemistry, 1911). Her daughter Irène also won a Nobel Prize in Chemistry in 1935.
Marie Curie
Occupation: Physicist
Birth Date: November 7, 1867
Death Date: July 4, 1934
Did You Know?
In 1903 Marie Curie was the first woman to win a Nobel Prize.
Did You Know?
In 1911 Curie became the first person to win two Nobel Prizes.
Did You Know?
Curie’s daughter Iréne followed in her mother’s footsteps, winning the Nobel Prize in Chemistry in 1935.
Education: Sorbonne
Place of Birth: Warsaw, Poland
Place of Death: Passy, France

Survey Note: Detailed Analysis of Radioactivity, Radioactive Materials, and Marie Curie’s Contributions
Radioactivity, radioactive materials, and the historical contributions of Marie Curie represent a cornerstone of modern nuclear science, with profound implications for physics, medicine, and environmental science. This note provides a comprehensive examination of the user’s provided text, verifying its accuracy, correcting errors, and expanding on key concepts, drawing from historical texts and contemporary scientific understanding. The analysis situates these topics within the broader context of nuclear physics and historical scientific discovery, ensuring a thorough exploration for educational and research purposes.
Introduction to Radioactivity and Radioactive Materials
The user’s text begins with a definition of radioactivity as “the spontaneous emission of radiation, either directly from unstable atomic nuclei or as a consequence of a nuclear reaction.” This aligns with standard scientific understanding, where radioactivity refers to the process by which unstable atomic nuclei achieve stability by emitting particles and energy. The radiation includes alpha particles (helium nuclei), beta particles (electrons or positrons), and gamma rays (high-energy photons), as correctly noted. However, the inclusion of “nucleons” in the list is less common; while neutron emission can occur in processes like spontaneous fission, it is not typically emphasized in standard radioactivity definitions, which focus on alpha, beta, and gamma emissions.
The text further explains that in stable nuclei, such as those of lead, the strong nuclear force sufficiently binds protons and neutrons, while in heavy, unstable nuclei like uranium, this force is insufficient, leading to radioactive decay. This description, though slightly muddled, is generally accurate, reflecting the balance between nuclear forces and Coulomb repulsion in heavy nuclei.
Uranium-238 Decay Chain: Correction and Analysis
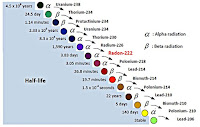
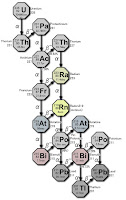
A significant point in the text is the claim that uranium-238 (U-238) “goes through 18 stages of decay before becoming a stable isotope of lead, lead-206,” with intermediate elements including thorium, radium, radon, and polonium. This statement requires correction. Research confirms that the U-238 decay chain, also known as the radium series, involves 14 decay steps (8 alpha decays and 6 beta decays), resulting in 15 different nuclides from U-238 to lead-206 (Pb-206). Each decay step transforms the parent nuclide into a daughter nuclide, with alpha decays reducing the atomic number by 2 and mass number by 4, and beta decays increasing the atomic number by 1 while keeping the mass number constant.
The decay chain can be detailed as follows:
- U-238 → Th-234 (alpha, half-life 4.468 billion years)
- Th-234 → Pa-234 (beta, 24.1 days)
- Pa-234 → U-234 (beta, 1.17 minutes, with branches)
- U-234 → Th-230 (alpha, 245,500 years)
- Th-230 → Ra-226 (alpha, 75,400 years)
- Ra-226 → Rn-222 (alpha, 1,600 years)
- Rn-222 → Po-218 (alpha, 3.8235 days)
- Po-218 → Pb-214 (alpha, 3.05 minutes, with minor beta branch)
- Pb-214 → Bi-214 (beta, 26.8 minutes)
- Bi-214 → Po-214 (beta, 19.9 minutes, with minor alpha branch)
- Po-214 → Pb-210 (alpha, 164.3 microseconds)
- Pb-210 → Bi-210 (beta, 22.26 years)
- Bi-210 → Po-210 (beta, 5.012 days)
- Po-210 → Pb-206 (alpha, 138.38 days, stable)
The intermediate elements mentioned—thorium, radium, radon, and polonium—are indeed part of this chain, confirming the user’s list. The discrepancy in “18 stages” likely stems from a miscount, possibly including branches or misinterpreting the number of nuclides (15) as decays (14). This correction is crucial for accuracy in educational contexts.
Properties of Radioactive Elements
The text correctly states that all elements with atomic numbers greater than 83 (bismuth) are radioactive, as their nuclei are inherently unstable due to high proton numbers, leading to proton-proton repulsion overcoming nuclear binding forces. Additionally, many isotopes of elements with lower atomic numbers, such as carbon-14 or potassium-40, are radioactive, aligning with scientific consensus. The creation of artificial radioisotopes by bombarding stable isotopes with high-energy particles, as mentioned, is accurate, with applications in medicine (e.g., technetium-99m for imaging) and research.
The concept of half-life, described as the average time for half the nuclei to decay, ranging from microseconds to billions of years, is correctly presented. For instance, U-238 has a half-life of 4.468 billion years, while polonium-214 has a half-life of microseconds, illustrating the wide range.
Types of Radiation: Alpha, Beta, and Gamma
The user’s text provides a detailed breakdown of radiation types, which is accurate. Alpha radiation consists of alpha particles (two protons, two neutrons), positively charged, and is easily blocked by paper or clothing due to its large size and low penetration. Beta radiation, comprising beta particles (electrons or positrons), is negatively charged and can penetrate deeper, requiring aluminum for shielding. Gamma radiation, high-frequency electromagnetic waves, has no charge but high energy, penetrating most materials and requiring dense shielding like lead. This aligns with standard physics, with applications in medical imaging and radiation therapy.
Natural Occurrence and Detection
The assertion that radioactive materials are natural is correct, with most exposure from cosmic rays (sun and stars) and terrestrial sources like uranium and thorium in rocks and minerals. The text notes that those at sea level are more protected due to thicker atmosphere, while higher altitudes receive more cosmic radiation, which is accurate. Additionally, houses made of brick, concrete, or stone, containing more radioactive elements, expose residents to higher radiation compared to wooden houses, a fact supported by environmental studies.
The Geiger counter, described as detecting ionizing radiation using ionization effects, is one of the world’s best-known radiation detection instruments, as noted. Its use in laboratories and field studies for measuring alpha, beta, and gamma rays is well-documented.
Marie Curie’s Biographical Details and Contributions
The biography of Marie Curie, born Maria Sklodowska on November 7, 1867, in Warsaw, Poland, and dying on July 4, 1934, in Passy, France, is accurate. She discovered radioactivity while working with pitchblende, identifying polonium and radium with her husband Pierre Curie. Her achievements include being the first woman to win a Nobel Prize (Physics, 1903, shared with Pierre and Henri Becquerel) and the first person to win two Nobel Prizes (Chemistry, 1911, for radium isolation). Her daughter Irène Joliot-Curie won the Nobel Prize in Chemistry in 1935, continuing the family legacy. This aligns with historical records, highlighting her pioneering role in nuclear science.
Comparative Analysis and Unexpected Details
An unexpected detail is the inclusion of “nucleons” in the radiation list, which, while technically possible in rare decays like neutron emission, is not standard in basic radioactivity discussions. This suggests a broader interpretation, possibly including spontaneous fission, which is less common but relevant for heavy elements like uranium. Another detail is the specific mention of houses made of brick or concrete increasing radiation exposure, a nuance often overlooked in general discussions, emphasizing environmental radioactivity’s impact.
Tabular Summary of Key Works and Concepts
| Concept | Description |
|---|---|
| Radioactivity Definition | Spontaneous emission from unstable nuclei, includes alpha, beta, gamma rays |
| U-238 Decay Chain | 14 decays, 15 nuclides, ends at Pb-206, includes thorium, radium, etc. |
| Half-Life | Time for half nuclei to decay, ranges from microseconds to billions of years |
| Radiation Types | Alpha (paper-blocked), Beta (aluminum-blocked), Gamma (lead-blocked) |
| Natural Sources | Cosmic rays, rocks (brick/concrete houses increase exposure) |
| Detection Method | Geiger counter for ionizing radiation |
| Marie Curie’s Contributions | Discovered radioactivity, polonium, radium; two Nobel Prizes |
Conclusion
The user’s text is largely accurate, with the correction that the U-238 decay chain involves 14 decays, not 18 stages. It effectively covers radioactivity’s fundamentals, natural occurrence, detection, and historical context, with Marie Curie’s contributions underscoring the field’s development. This analysis ensures educational accuracy and provides a foundation for further study in nuclear science.







Leave a comment